Volume of distribution and Clearance are fundamental Pharmacokinetic Parameters
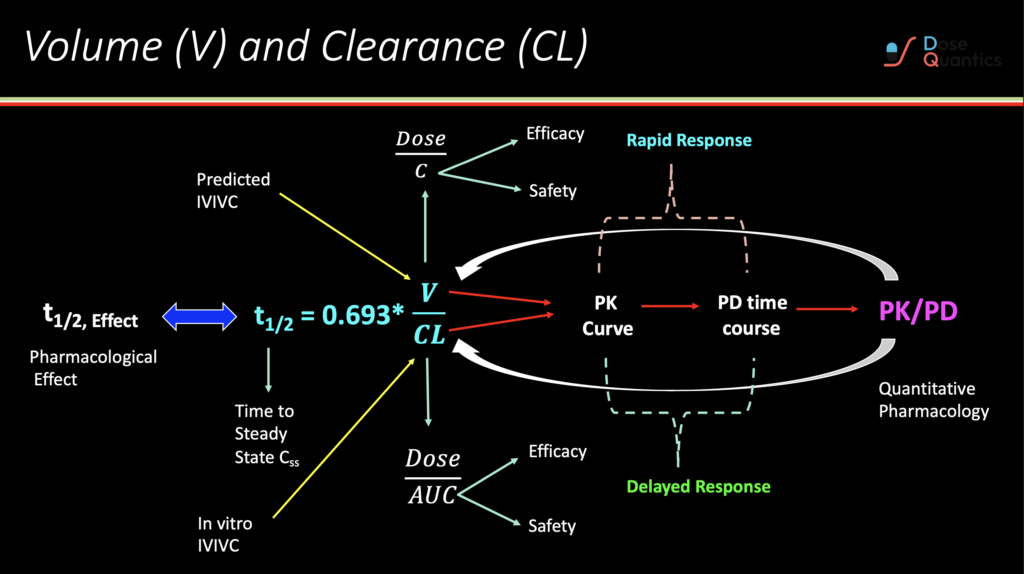
Volume of distribution (V) and Clearance (CL) are fundamental pharmacokinetic (PK) parameters that determine the PK properties of a molecule. V is dependent on the physico-chemical properties and affinity of binding to tissues and the CL is dependent on the concentration, blood flow and biochemical mechanism of elimination of the molecule.
V is predicted using lipophilicity, protein binding and in silico methods. CL is predicted using metabolic stability and transporter assays. V and CL are estimated in vivo in PK studies. The in vitro to in vivo correlations (IVIVC) are explored for both the parameters with a goal to optimize both for the desired PK properties.
In discovery programs the PK is optimized based on parameters such as solubility, lipophilicity, PPB, metabolic stability, transporter, permeability. However, the PK properties that are optimal for efficacy is not known unless the relationship between the molecule’s PK (concentration time course) and its pharmacological action (pharmacodynamics). Data from PK/PD experiments provide information on what PK properties have to be optimized to improve efficacy and safety.
Modifying molecular structure can lead to changes in both CL and V, which in turn determine its PK properties. Relating the concentration-time (PK) to effect-time and effect-concentration help in quantitating pharmacology of the molecule such as the onset, intensity and duration of effect.
The magnitude of V and CL required for efficacy can be obtained from PK/PD studies that can in turn inform optimization programs for setting criteria for evaluation.
Ramesh Jayaraman,
Director
DoseQuantics Consulting
www.dosequantics.com
