Physiologically Based Pharmacokinetic (PBPK) Modelling in Drug Discovery and Development
Physiologically based pharmacokinetics (PBPK) describes the pharmacokinetics (PK) of a drug by integrating its physicochemical and in vitro ADME properties (drug specific) with physiological parameters (system specific) of the body. This is known as the” bottom up” approach (mechanistic), the opposite of empirical description of PK (sum of exponentials/compartments) known as “top down” approach. The mechanistic basis of PBPK enables prediction of PK profiles in humans and preclinical species. PBPK modelling and simulation has become an integral part of drug development (Model Informed Drug Development –MIDD)– e.g. prediction of first time in human dose, drug-drug interactions, doses in different populations. PBPK modelling is being used for decision making by regulatory agencies – e.g. waiver of clinical trials, labelling information, optimal design of clinical trials.
The strength of a PK model is evaluated by its ability to predict the behaviour of the drug at a dose or regimen that is different from the dose for which the model was developed. When the model successfully predicts PK across dose levels or regimens its robustness and reliability increase.
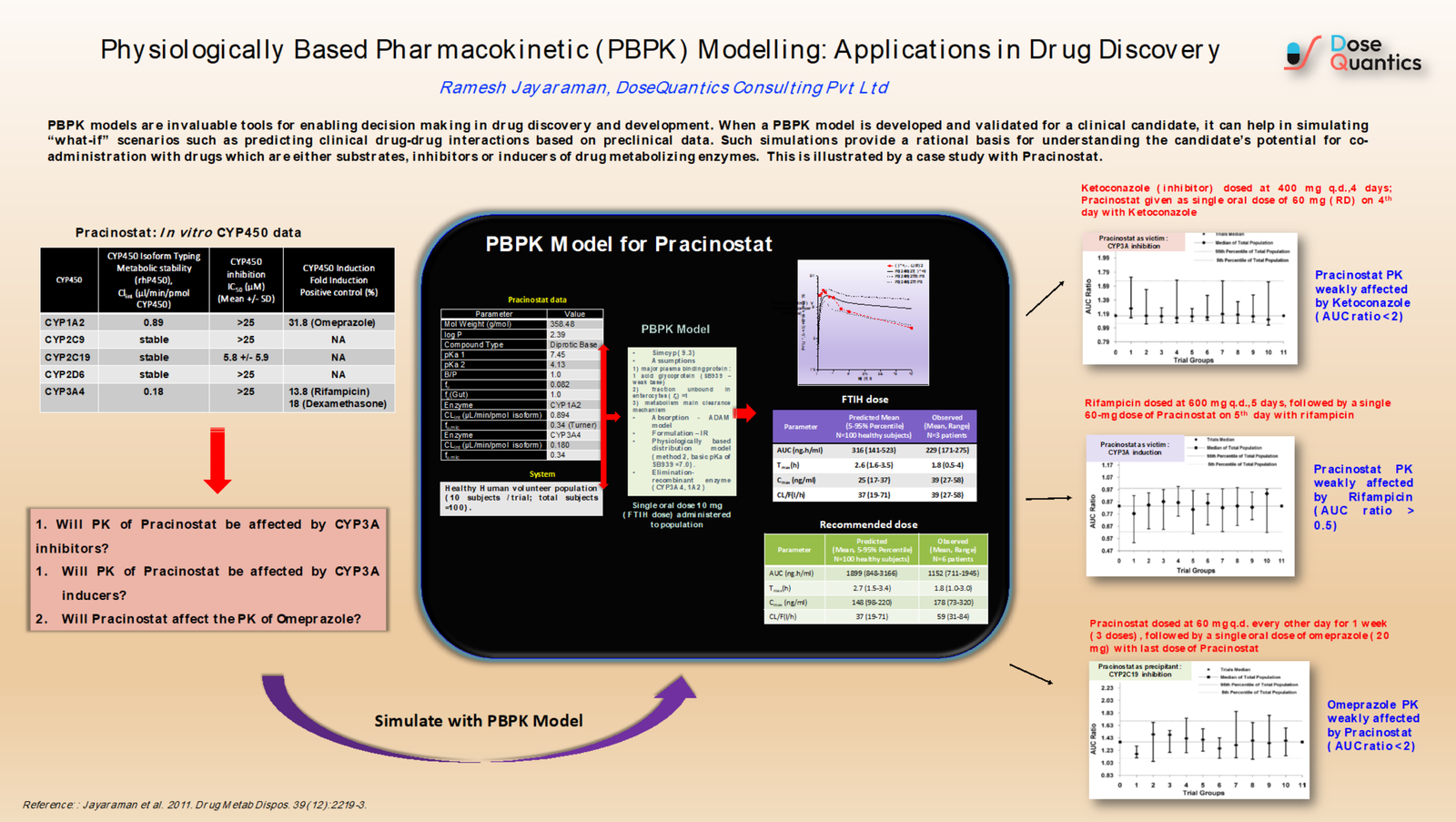
PBPK models are invaluable tools for enabling decision making in drug discovery and development. When a PBPK model is developed and validated for a clinical candidate, it can help in simulating “what-if” scenarios such as predicting clinical drug-drug interactions based on preclinical data. Such simulations provide a rational basis for understanding the candidate’s potential for co-administration with drugs which are either substrates, inhibitors or inducers of drug metabolizing enzymes. This is illustrated by a case study with Pracinostat.
- Ramesh Jayaraman
DoseQuantics Consulting Pvt Ltd
References
- Jayaraman et al. 2011. Preclinical metabolism and disposition of SB939 (Pracinostat), an orally active histone deacetylase inhibitor, and prediction of human pharmacokinetics. Drug Metab Dispos. 39(12):2219-32
