Antibacterial drug activity can be classified as concentration dependent or time dependent
Ramesh Jayaraman, DoseQuantics Consulting Pvt Ltd.
7th September, 2025
Recently, two letters to the editors appeared in the journal Antimicrobial Agents Chemotherapy (1, 2) that discussed the merits of classification of anti-bacterial drugs as bacteriostatic and bactericidal. Spellberg et al observed that this classification is not correct and does not have clinical utility because there is no significant difference in clinical efficacy between bacteriostatic and bactericidal drugs (1). They also go on to state that the so called static antibiotics show bacterial killing and that there is no single antibiotic drug that is static which does not kill. I agree Spellberg et al on all the counts.
The antibacterial effect of a drug depends on the growth rate of the bacteria and the killing rate of the bacteria by the drug at a specific concentration.
The definitions of MIC (Minimum Inhibitory Concentration) and MBC (Minimum Bactericidal Concentration) are related to the activity of an antibacterial drug at a specific concentration. MIC is the concentration at which there is no visible growth after 24 hours and MBC is the concentration at which there is a 1000 fold (99.9%) reduction in bacterial numbers after 24 h. The classification of a drug as a static or cidal agent is based on the ratio of MBC to its MIC (Static = MBC/MIC > 4; Cidal = MBC/MIC < 4) (1). Clearly, the definitions of static and cidal are concentration dependent. In other words a drug that is static at one concentration can be cidal at a higher concentration whether the ratio is greater or lesser than 4. Indeed, it has been clearly shown that antibacterial drugs display two distinct types of killing activity over time (3,4).
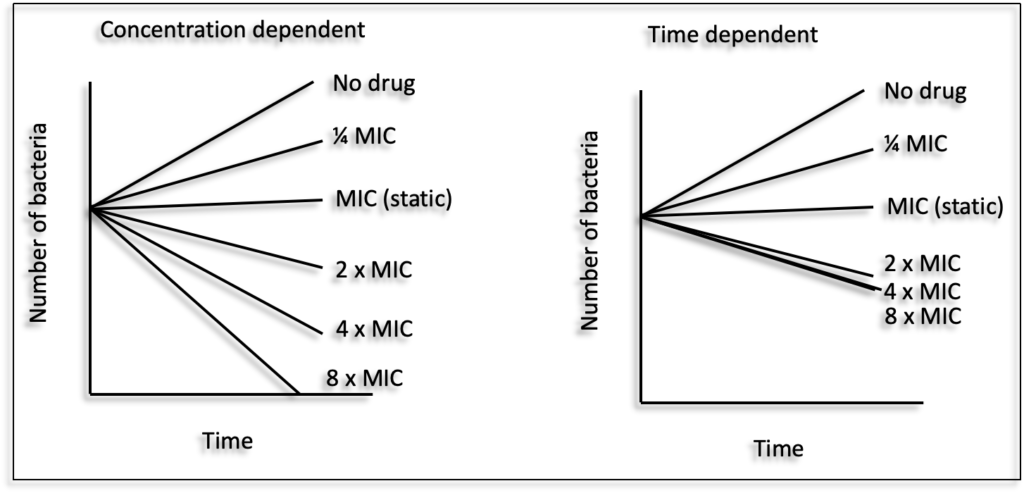
Antibacterial drugs are classified into two major categories based on their in vitro killing of bacteria (killing kinetics), in presence of increasing concentrations (multiples of their minimum inhibitory concentration (MIC)), over 24 hours :
1. Concentration dependent killing : there is an increase in the bacterial killing rate and magnitude over a wide range of drug concentrations (e.g. Fluoroquinolones, Aminoglycosides);
2. Time dependent (or minimum concentration dependent) killing : the killing rate increases over a limited range of concentrations and reaches saturation (no further increase in magnitude of kill) (e.g. beta-lactams, cephalosporins, carbapenems) (3, 4). For such drugs higher magnitudes of kill can be achieved by exposing bacteria at the saturating concentration for a longer period of time. Thus, for concentration dependent drugs, maximizing concentrations yields maximum efficacy and for time dependent drugs maximizing time exposure, rather than concentration, yields maximum efficacy.
The patterns of in vitro killing of drugs show similarities with their in vivo killing patterns in animal models of infection (4). For several classes of antibacterial drugs, when in vitro killing kinetics and MIC is combined with their pharmacokinetics (PK), a high degree of correlation was observed with their efficacy or pharmacodynamics (PD) in a wide range of animal models of infection and in the clinic (5, 6). Based on the above observations, the efficacy of antibacterial drugs is described in terms of the ratio of a PK parameter and the MIC known as the PK/PD index. The three main PK/PD indices associated with antibacterial efficacy are the ratio of free maximum drug concentration in plasma and MIC (fCmax/MIC), ratio of free drug area under the plasma concentration time curve and MIC (fAUC/MIC) and the percentage of time the free drug concentration exceeds the MIC in a twenty four hour dosing interval (%fT >MIC). For example, the PK/PD index associated with efficacy of fluoroquinolone drugs is fAUC/MIC whereas the PK/PD index associated with the efficacy of beta lactam drugs is (%fT >MIC).
The PK/PD index determines the rationale for dose and regimen in the clinic (6). For fAUC/MIC drugs, the goal is to maximise dose (exposure) and for %fT>MIC the goal is to frequently dose to maintain concentrations above a certain threshold. For any antibacterial drug, the in vitro killing pattern, MIC and PK (PK/PD index and magnitude) is generally predictive of the success or failure of clinical response against a variety of bacterial pathogens and different sites of infections (7). Taken together, the classification of antibacterial drugs based on relationship between the killing pattern and concentration over time is better descriptive of their characteristics and has high clinical utility.
I suggest classifying antibacterial drugs as concentration dependent or time dependent instead of static or cidal.
References
- Spellberg B, Wald-Dickler N, Holtom P, Meyer-Sautter P, Camp A, Diaz AD, Buhamad R, Vazquez ASM, Aguirre-Garcia GM, Stanton M, Butler-Wu SM, Chiu I, Ergenc Z, Bhoojhawon G, Murri R, Maraolo AE, Cabanilla G, Riccardi N, Tshisevhe V, Behenna C, Williams KS, Kufel WD, Wojciaczyk N, Pimentel BV, Muyidi A, Costa RP, Motta F, Bortolussi-Courval É, Lee TC, McDonald E, Ghanem B, Nelson Z. 2025 . Static vs. cidal: it’s not complex; it’s simply incorrect. Antimicrob Agents Chemother. 6;69(8)
- Gil-Gil T, Berryhill BA. 2025. Reply to Spellberg et al., “Static vs cidal: it’s not complex; it’s simply incorrect”. Antimicrob Agents Chemother. 6;69(8)
- Vogelman B, Craig WA. 1986. Kinetics of antimicrobial activity. J Pediatr. 108 (5 Pt 2):835-40.
- Craig WA. 2001. Does the dose matter? Clin Infect Dis. 15:33 (Suppl 3:S233-7).
- Craig, WA. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 26(1):1-10
- Ambrose PG, Bhavnani SM, Rubino CM, Louie A, Gumbo T, Forrest A, Drusano GL. 2007. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it’s not just for mice anymore. Clin Infect Dis. 44 (1):79-86.
- Ambrose PG, Bhavnani SM, Ellis-Grosse EJ, Drusano GL. 2010. Pharmacokinetic-pharmacodynamic considerations in the design of hospital-acquired or ventilator-associated bacterial pneumonia studies: look before you leap! Clin Infect Dis. 1:51 (Suppl 1:S103-10)
