Pacritinib (VONJO) shows a relationship between objective response and meaningful clinical benefit in Myelofibrosis patients
Ramesh Jayaraman, DoseQuantics Consulting,
September 12th 2025
The true worth of an anti-cancer drug is measured not just by its ability to reduce the cancer burden aka objective response (reduction in tumor size, prevention of metastasis) but the relationship between the reduction in cancer burden and the increase in meaningful clinical benefit (increase in overall survival of the patient associated with increased quality of life).
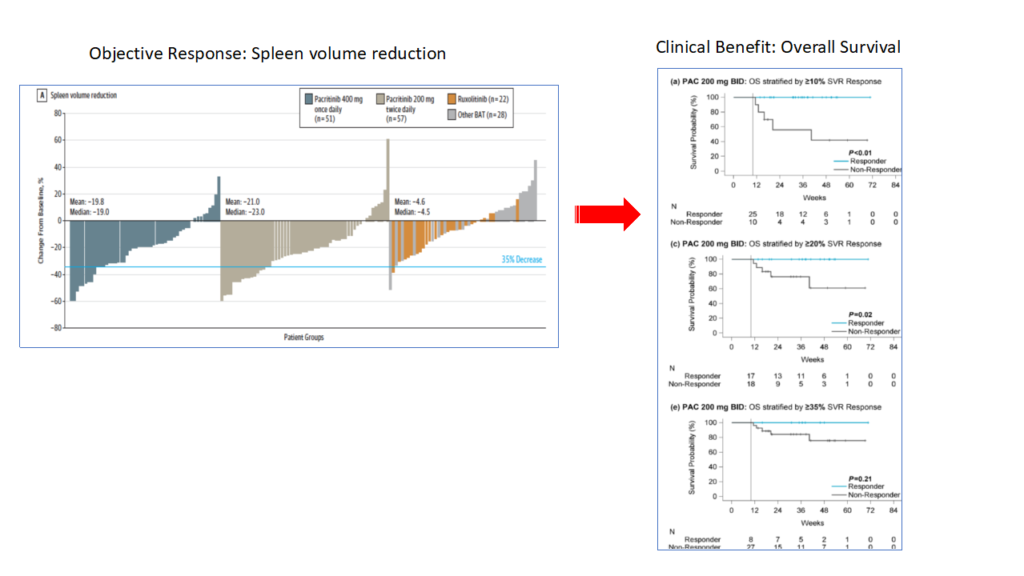
Pacritinib (VONJO), a JAK2, IRAK1, ACVR inhibitor, was approved for the treatment high risk myelofibrosis patients with platelet count (< 50 x 109/L), based on its ability to significantly reduce spleen volume by more than 35% in pivotal phase 3 trials. However, whether that translated into clinical benefit (overall survival) was not known.
In a recent post hoc analysis of phase 3 clinical trial data collected from patients, Pacritinib demonstrated improved OS in patients whose spleen volume reduction was > 10% when compared to patients where it did not show spleen volume reduction (SVR <10 % reduction). This correlation was also seen when improvement in Total Symptom Score (TSS) was used as an objective response.
In summary, the improvement in objective response in myelofibrosis patients treated with Pacritinib (200 mg, twice daily, orally) was correlated with improved overall survival. It appears that SVR and TSS could serve as surrogate markers of OS for Pacritinib.
References:
Ajufo H, Bewersdorf JP, Harrison C, Palandri F, Mascarenhas J, Palmer J, Gerds A, Kiladjian JJ, Buckley S, Derkach A, Roman-Torres K, Rampal RK. Pacritinib Response Is Associated With Overall Survival in Myelofibrosis: PERSIST-2 Landmark Analysis of Survival. Eur J Haematol. 2025 Feb;114(2):238-247. doi: 10.1111/ejh.14321. Epub 2024 Oct 14
Mesa RA, Vannucchi AM, Mead A, Egyed M, Szoke A, Suvorov A, Jakucs J, Perkins A, Prasad R, Mayer J, Demeter J, Ganly P, Singer JW, Zhou H, Dean JP, Te Boekhorst PA, Nangalia J, Kiladjian JJ, Harrison CN. 2017. Pacritinib versus best available therapy for the treatment of myelofibrosis irrespective of baseline cytopenias (PERSIST-1): an international, randomised, phase 3 trial. Lancet Haematol.;4(5):e225-e236. doi: 10.1016/S2352-3026(17)30027-3. Epub 2017 Mar 20.
Mascarenhas J, Hoffman R, Talpaz M, Gerds AT, Stein B, Gupta V, Szoke A, Drummond M, Pristupa A, Granston T, Daly R, Al-Fayoumi S, Callahan JA, Singer JW, Gotlib J, Jamieson C, Harrison C, Mesa R, Verstovsek S. 2018. Pacritinib vs Best Available Therapy, Including Ruxolitinib, in Patients With Myelofibrosis: A Randomized Clinical Trial. JAMA Oncol. ;4(5):652-659. doi: 10.1001/jamaoncol.2017.5818.
