Prediction of Human Pharmacokinetics and Estimation of First Time in Human Dose
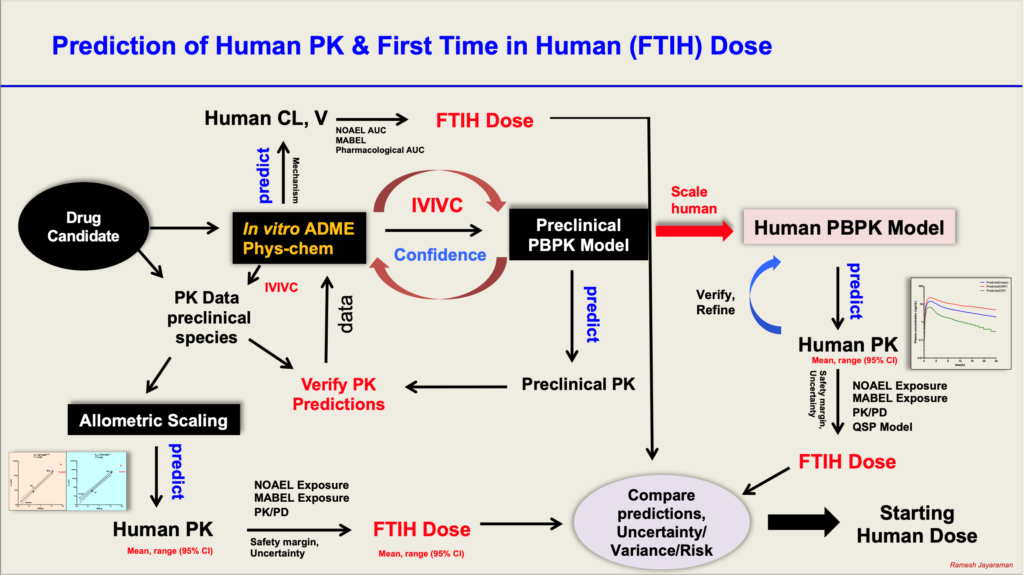
For any drug discovery company, a major milestone is the administration of the clinical drug candidate for the first time in humans. The management, Project members including the pharmacometricians in particular wait anxiously to see how the first patient reacts to the first dose (potential adverse effects) and what the PK of the CD will be. Any signs of unexpected adverse toxicity or failure to achieve the desired PK can lead to halting the program, which can impact companies significantly.
The probability of success for a clinical drug candidate from first time in human dose to marketing approval is approximately 10 %. The probability of success increases with progressive clinical stages.
Important reasons for failure in phase 1 clinical trials are toxicity and failure to achieve the pharmacokinetics required for pharmacological effect (efficacy/pharmacodynamics). Clearly it is imperative to understand the potential of a clinical drug candidate to achieve the desired PK associated with safety and efficacy before it enters clinical testing for the first time in humans.
Human PK parameters and PK profiles can be predicted using different methods:
a) in vitro –in vivo scaling of Clearance and Volume of Distribution using in vitro ADME data – well characterized data required. Very useful method when there is no access to PBPK models.
b) Allometric scaling using PK data from preclinical species: Predictions should carry mean and 95% confidence intervals for both IV and oral predictions. Oral predictions are challenging because of variable oral bioavailability. In such cases IV data can be used along with variable F to predict a range of CL/F and V/F. Very useful method when there is no access to PBPK models.
c) Physiologically Based Pharmacokinetic (PBPK) Modelling: Mechanistic and bottom up approach. Quality and well characterized in vitro ADME data are essential as they form the input data. Predicts PK parameters and profiles based on population characteristics and gives mean and variance. This can be used to simulate virtual clinical trials to evaluate what if scenarios. Can be integrated with PD and QSP models to predict PK/PD in humans.
All the three methods can be used simultaneously to get an idea of comparative estimates of PK parameters in humans.
The predicted PK parameters, estimated using each of the above methods, can be compared with data from in vitro safety pharmacology, nonclinical toxicology (Dose-exposure; NOAEL, MABEL), and nonclinical pharmacology (Dose-exposure-response, PK/PD relationships) to estimate a safe starting dose in humans (FTIH dose).
The predictions from various methods should be evaluated in an integrated manner to understand the strengths and weaknesses of the predictions, along with potential variability in different populations, uncertainties associated with predictions. The predicted mean and range of doses should be reported and considered to enable decisions for choice of FTIH dose selection.
The metrics of accurate predictions are usually estimated as ≤ 2 fold differences between observed and predicted values. But one cannot predict this in a prospective manner. Successful predictions can also be evaluated based on the mean observed data falling within a predicted range.
Some companies and researchers share their experiences and analysis for predictions and observed data. It would be nice to see both successful and unsuccessful predictions reported in literature, which can help in greater understanding of methods for FTIH predictions.
References:
- Boxenbaum H and DiLea C.1995. First-time-in-human dose selection: allometric thoughts and perspectives. J Clin Pharmacol 35:957–966.
- Obach RS, Baxter JG, Liston TE, Silber BM, Jones BC, MacIntyre F, Rance DJ, and Wastall P. 1997. The prediction of human pharmacokinetic parameters from preclinical and in vitro metabolism data. J Pharmacol Exp Ther 283:46 –58.
- Gabrielsson J and Weiner D. 2000. Pharmacokinetic and Pharmacodynamic data analysis: Concepts and Applications. 3rd Edition. Swedish Pharmaceutical Press.
- Zhao P, Zhang L, Grillo JA, Liu Q, Bullock JM, Moon YJ, Song P, Brar SS, Madabushi R, Wu, TC, et al. 2010. Applications of physiologically based pharmacokinetic (PBPK) modeling and simulation during regulatory review. Clin Pharmacol Ther 89:259 –267.
- Amin Rostami-Hodjegan, Masoud Jamei, & Thomas Polasek. PBPK’s Pivotal Role in Modern Drug Development: Busting Common Myths and Misconceptions. Certara White Paper 2019.
