Quantifying Pharmacokinetics
To quantify pharmacological and toxicological effects of a drug or a molecule (e.g. Efficacy = Emax or Imax; Potency =EC50 or IC50) based on dose and effect, it is essential to quantify its pharmacokinetics. Quantifying PK helps in understanding the relationship between pharmacokinetics (PK) and pharmacodynamics (PD) or PK/PD. Quantitative PK/PD (Quantitative Pharmacology) is the basis for setting dose, regimen and duration for treatment in nonclinical and clinical settings (2).
Methods of pharmacokinetic analysis for quantification of PK parameters:
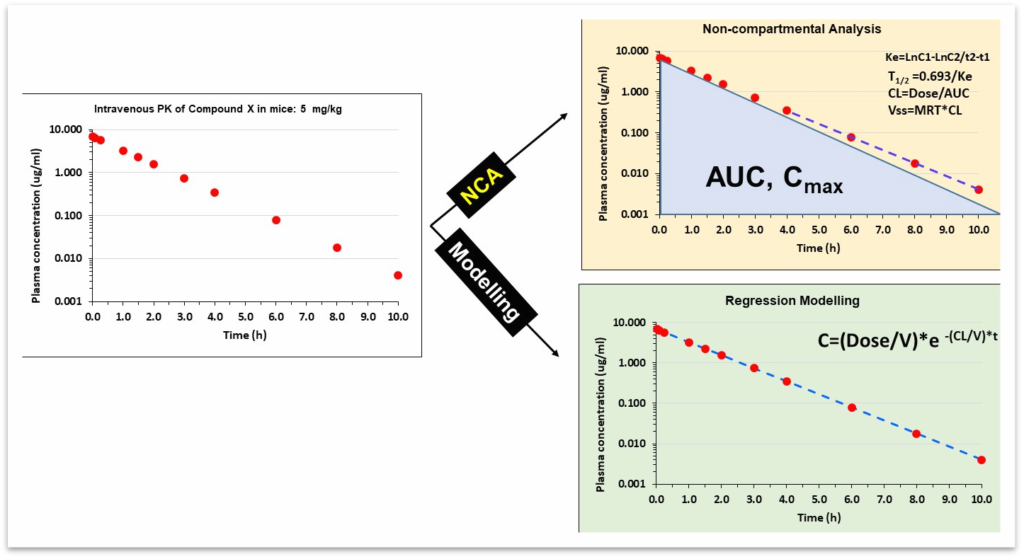
The PK of a molecule can be analyzed and quantified using
a) Non-compartmental analysis (NCA)
b) Non-linear regression modelling
NCA: This method uses the application of the trapezoidal rule for estimating the area under a plasma-concentration time curve. This method is applicable to first-order (linear) models and does not consider compartmental behavior.
Non-linear regression modelling: This method uses mathematical equations (exponentials or differential equations) to describe the PK in terms of compartmental behaviour. The primary parameters of the model are usually based on physiologically relevant quantities such as the volume of distribution and clearance.
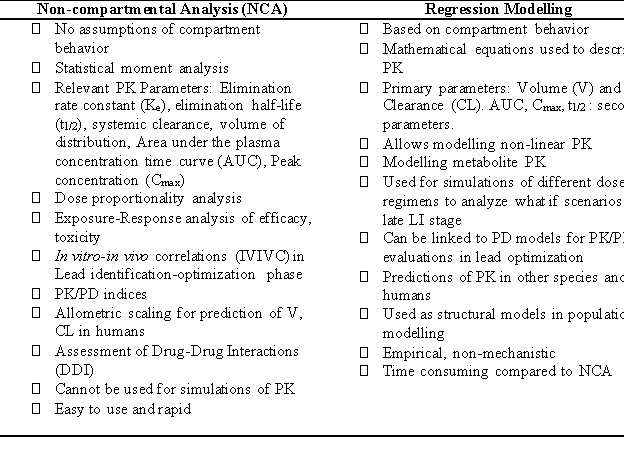
Application of NCA and Regression modelling in drug discovery and development for quantification of pharmacokinetics
References:
- Rowland, M and Tozer, T.N. Clinical Pharmacokinetics: concepts and applications. 3rd ed.
- Gabrielsson, J.; Green, A.R.; Quantitative pharmacology or pharmacokinetic pharmacodynamic integration should be a vital component in integrative pharmacology. J Pharmacol Exp Ther., 2009, 331(3), 767-74.
- Gabrielsson J and Weiner D. 2000. Pharmacokinetic and Pharmacodynamic data analysis: Concepts and Applications. 3rd Edition. Swedish Pharmaceutical Press.
Ramesh Jayaraman
