What is a Lead Molecule?
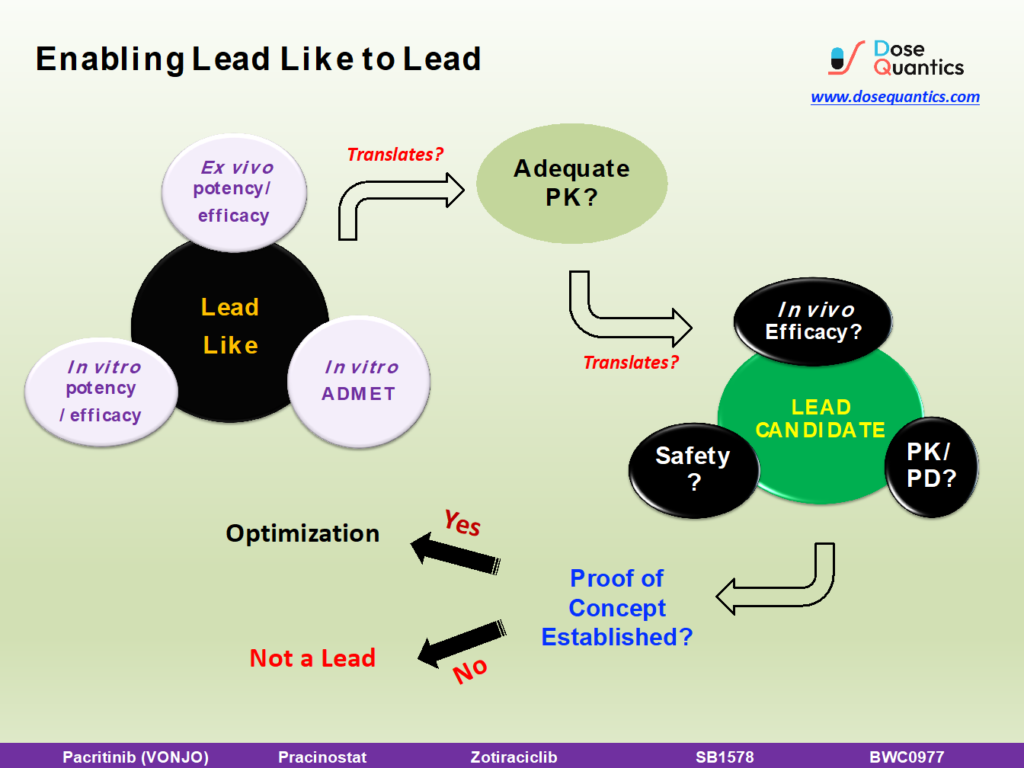
Most often a Lead is determined based on meeting project criteria for
- in vitro and ex vivo pharmacological potency/efficacy,
- in vitro ADMET properties
- in vitro safety margin
- Pharmacokinetics (in some cases this surprisingly is deemed not necessary!)
But, a vital component seems to be missing in this set of criteria – Efficacy and PK/PD OR is not given importance.
When all the above set criteria do not translate to efficacy (pharmacological effect/Pharmacodynamics) or safety, it suggests that there is no correlation between in vitro and in vivo Pharmacology. In other words, the proof of concept (PoC) has not been demonstrated. There is something missing or some factor which has not been accounted for in the lead compound. If the in vitro pharmacology, ADMET and PK of the compound do not demonstrate quantitative pharmacology (PK/PD), then it cannot be called as a Lead. In short, the project hypothesis failed.
When an in vitro in vivo (IVIV) relationship is shown for a Lead, then it offers a quantitative basis for optimization programs. Basically, it sets the stage with a certain set of in vitro parameters correlated quantitatively with in vivo parameters. Conversely, if there is absence of IVIV relationship for Pharmacological effect (lack of PK/PD correlation), on what basis will the lead optimization program run on?
When a program transitions to optimization with out proof of PK/PD and Efficacy, the basis for optimization is not based on sound PK/PD rationale. After all, all the in vitro parameters (be it pharmacological, ADME, Tox set as LI criteria) should be related to in vivo effect and safety.
